




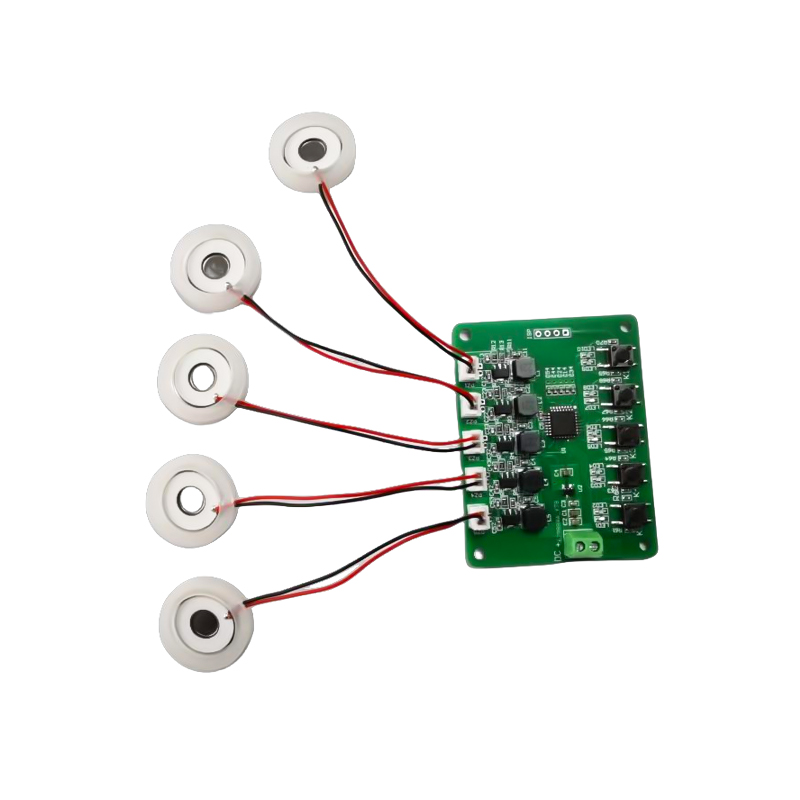




Medical Mesh Piezo Atomizer Disc Manufacturers












Medical Mesh Nebulizer Tablets are precision components used in medical devices to convert medications or solutions into tiny aerosol particles for the treatment of various respiratory disorders such as asthma, and chronic obstructive pulmonary disease (COPD), etc. by inhalation.
The design and material selection of the nebulizer sheet is critical to ensure effective drug delivery and patient comfort. The stainless diaphragm sheet is punched with thousands of tiny 2-3μm mesh holes in the steel sheet through laser drilling technology in a precision laser process, and then it is tightly bonded with the piezoelectric ceramic round sheet, which converts the resonant frequency of the piezoelectric sheet into high-frequency vibration energy through the output signal of the PCB driver board, and rapidly breaks down the liquid medication into fine aerosol particles. These droplets are also called median particle size, when the median particle size D50 percentage reaches >60% or more, the particles will be small enough to be able to reach deep into the lungs to the surface of the alveoli with breathing, thus improving the absorption efficiency of the medication and the therapeutic effect. Medical Mesh micro mesh nebulizer sheet mainly has several forms exist, stainless steel micro mesh nebulizer sheet, nickel palladium medical nebulizer sheet, PI polymer medical nebulizer sheet, and so on. All of the above are through the piezoelectric ceramic vibration energy conversion to achieve the atomization effect.
Saida Piezoelectric is a globally renowned technology-based enterprise specializing in the production and manufacturing of medical atomization related products, we are China Medical Mesh Piezo Atomizer Disc Manufacturers and Custom Portable Medical Mesh Piezo Atomizer Disc Company, We have been committed to the innovation, research and development, and promotion of ultrasonic piezoelectric ceramics, lead-free atomization products, electronic components, and related PCBA design solutions. Our goal is to improve people's quality of life through technology, and achieve a more efficient, green, and sustainable future for society.
Since its establishment in April 2011, the company has been at the forefront of technological development, with an outstanding research and development team, advanced production and manufacturing equipment, and precision testing equipment. We cover a wide range of ultrasonic atomization products in our professional field, including lead based and lead-free medical inhalable atomization tablets, humidifier atomization tablets, essential oil atomization tablets, industrial atomization tablets, as well as sensing products related to ultrasonic piezoelectric and PCBA driving solutions. We not only focus on in-depth technological research and development, but also actively apply technological achievements to practical life, so that technological products can truly serve and benefit society, making the world more exciting and beautiful.
Certification

Our company's medical inhalable piezoelectric atomization series products have all passed the certifications required for export standards. They include ROHS certification, ISO9001 quality system certification, health system certification GB/T45001-2020/ISO 45001:2018, GB/T28001-2001 idt OHSAS18001:1999 Occupational Health and Safety Certification, environmental system certification GB/T24001-2016/ISO 14001:2015, California Proposition 65, REACH, and the medical atomization products have also been certified by the U.S. FDA.
news
-
Industry news
What Are the Key Benefits of Using Piezo Atomization Chips...
Introduction In the medical device industry, innovations in technology continually enhance treatment effectiveness, safety, and comfort for patients. One such breakthrough is the integration of piezo atomization chips, w...
-
Industry news
How Do Humidifier Discs Work to Maintain Optimal Humidity ...
Humidity plays a vital role in creating a comfortable living environment, particularly in regions where dry air can lead to health problems such as dry skin, respiratory issues, and discomfort. Humidifiers are essential ...
-
Industry news
How Do Medical Piezoelectric Ceramic Discs Benefit Patient...
Introduction to Medical Piezoelectric Ceramic Discs In recent years, advancements in non-invasive medical treatments have led to the introduction of innovative technologies that offer patients effective and minimally dis...
Medical Mesh Piezo Atomizer Industry Knowledge Extension
How does the design of medical mesh piezoelectric atomizer meet the strict standards and requirements of the medical industry?
Since its establishment in 2011, Haining Saida Piezoelectric Ceramics Co., Ltd. has always been at the forefront of the development of ultrasonic atomization technology. With its excellent R&D team, advanced production equipment and precision testing equipment, it focuses on the R&D and production of ultrasonic atomization products. The company's products cover a wide range of fields including medical inhalation atomizers, humidifier atomizers, essential oil atomizers, industrial atomizers, ultrasonic piezoelectric sensors and PCBA drive solutions. The company not only focuses on in-depth technology research and development, but also actively applies technological achievements to real life, so that scientific and technological products can truly serve the society and make the world a better place.
When designing medical mesh piezoelectric atomizers, Haining Saida Piezoelectric Ceramics Co., Ltd. took the following measures to meet the strict standards and requirements of the medical industry:
1. Strictly follow medical industry standards
The design of medical mesh piezoelectric atomizers strictly follows international and domestic medical industry standards, such as ISO 13485 (Quality Management System for Medical Devices), FDA (US Food and Drug Administration) and other relevant standards. These standards ensure that the products meet the high quality and safety requirements of medical devices during the design, production and testing process.
2. Selection of biocompatible materials
The company is well aware of the particularity of medical products in direct contact with the human body, so it is extremely cautious in material selection. Medical mesh piezoelectric atomizers use medical-grade high-purity ceramic materials to ensure that they will not release harmful substances during use, and have good biocompatibility to avoid irritation or allergic reactions to the human body.
3. Precision mesh design and optimization of atomization effect
The core of medical mesh piezoelectric atomizers lies in their mesh structure. The size and distribution of these meshes directly affect the atomization effect. The company uses advanced design and manufacturing processes to precisely control the size and arrangement of the meshes to ensure the uniformity and consistency of the atomized particles. For example, the particle size of the atomized particles can be controlled within the range of 1-5 microns, which is essential for the effective delivery and absorption of drugs.
4. Strict quality control and testing process
During the production process, the company uses precision testing equipment to conduct comprehensive quality testing on medical mesh piezoelectric atomizers. This includes material performance testing, atomization effect testing, durability testing, and biocompatibility testing. Through these rigorous testing processes, it is ensured that each atomizer meets the high standards of the medical industry.
5. Customized solutions and customer feedback mechanism
Haining Saida Piezoelectric Ceramics Co., Ltd. provides customized medical mesh piezoelectric atomizer solutions, which can adjust product parameters according to the specific needs of customers. At the same time, the company has established a complete customer feedback mechanism. Through close cooperation with medical institutions and equipment manufacturers, it continuously collects usage feedback, optimizes product design, and ensures that products can meet the actual needs of the medical industry.



 English
English 中文简体
中文简体








